NMDA Receptor Overview
Neurotransmission in the brain involves a dynamic balance between excitatory (activating) and inhibitory (dampening) signals exchanged among neurons. Excitatory signaling is primarily mediated by the release of glutamate and the activation of glutamate-sensitive receptors. Among these, NMDA receptors form a specialized subclass that require glutamate, glycine, and prior neuronal depolarization for activation. This specialized activity is essential for neurological development, synaptic plasticity, learning and memory, and the fine-tuning of excitatory/inhibitory balance within neural circuits.
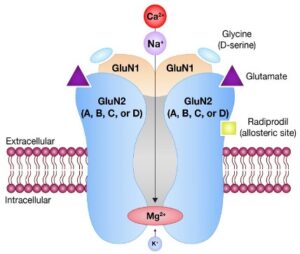
Functional NMDA receptors are heterotetramers composed of two GluN1 subunits paired with two GluN2 subunits, typically of the GluN2A, GluN2B, GluN2C, or GluN2D subtypes. These subunits are encoded by GRIN genes (GRIN1, GRIN2A, GRIN2B, GRIN2C, and GRIN2D). Channel activation requires glycine binding to the GluN1 subunits and glutamate binding to the GluN2 subunits, which together relieve the magnesium block within the channel pore, to allow for the flow of sodium and calcium cations into the cell.
NMDA receptor dysfunction in neurological disease
Dysfunctional NMDA receptor activity has been implicated in a broad range of neurological and neuropsychiatric conditions, including epilepsy, neurodevelopmental disorders, Alzheimer’s Disease, and major depressive disorder. Alterations in NMDA receptor function are associated with seizures, developmental delays, and behavioral dysregulation in developmental epilepsies and encephalopathies and other neurodevelopmental disorders. GRIN-related neurodevelopmental disorder (GRIN-NDD) refers to a spectrum disorder caused by de novo mutations in GRIN1, GRIN2A, GRIN2B, or GRIN2D genes, which produce pathological variants that alter NMDA receptor activity - ranging from gain-of-function to loss-of-function, depending on the specific mutations.
NMDA receptor modulation as a therapeutic strategy
Therapeutics targeting NMDA employ various strategies, such as blocking the channel pore, preventing agonist binding to inhibit activity, or mimicking agonist binding to enhance activation. In contrast, allosteric modulators, bind to distinct sites from both the channel pore and the agonist binding domains. These modulators offer a therapeutic approach that allows for fine-tuning of NMDA receptor activity without directly opening the channel or fully inhibiting its function.
Genetic testing and neurodevelopmental disorders
As the cost of diagnostic techniques like whole genome sequencing (WGS) / whole exome sequencing (WES) has decreased and patient access has expanded, research is increasingly uncovering the complex genetic origins of neurodevelopmental disorders. GRIN Therapeutics is partnering with companies, researchers, and academic groups to ensure accurate, up-to-date genetic characterization is available to patient communities.
Resources:
Clinical Trial Programs
GRIN-NDD

GRIN-related neurodevelopmental disorder (GRIN-NDD) is caused by de novo mutational variants in GRIN1, GRIN2A, GRIN2B, and GRIN2D genes, which encode subunits of the NMDA receptor. This rare condition can have devastating impacts on patients and their families; developing safe and effective treatments for GRIN-NDD is a significant unmet need. GRIN Therapeutics is currently developing radiprodil, an oral, selective, negative allosteric modulator of the GluN2B NMDA receptor subunit as an interventional strategy that targets the underlying pathology of the disease. The Honeycomb Phase 1b/2a trial enrolled 15 GRIN-NDD patients to evaluate the preliminary safety and efficacy of radiprodil, results were deemed sufficient to warrant a larger, Phase 3 trial, Beeline, which is currently enrolling.
For additional information about the Honeycomb Study, visit thehoneycombstudy.com or clinicaltrials.gov. Early results were presented at the 2024 AES meeting (link to abstract)

GRIN-related neurodevelopmental disorder (GRIN-NDD) arises from de novo mutations in GRIN1, GRIN2A, GRIN2B, and/or GRIN2D genes, which encode subunits of the NMDA receptor. This rare condition can have profound and debilitating effects on patients and their families. There is a significant unmet need for safe, effective and targeted treatments for GRIN-NDD. The Honeycomb Phase 1b/2a trial investigated radiprodil, an oral, selective negative allosteric modulator of the GluN2B-subunit of the NMDA receptor, in GRIN-NDD patients with gain-of-function (GoF) variants. Results from the study showed potentially disease-modifying improvements.
Beeline is our global Phase 3 trial evaluating radiprodil in GRIN-NDD individuals with GoF variants. This two-arm, placebo-controlled, multi-center study assesses radiprodil as add-on therapy to standard of care.
For additional information about the Beeline study, visit clinicaltrials.gov.
GRIN-related neurodevelopmental disorder (GRIN-NDD) was first characterized in 2010 as a condition resulting from de novo mutational variants in GRIN1, GRIN2A, GRIN2B, and/or GRIN2D genes, which encode subunits of the NMDA receptor. This disorder presents with a range of neurological symptoms, including seizures, intellectual disability, autism spectrum disorder, behavioral dysregulation, developmental delay, motor dysfunction, hypotonia, sleep disturbances, and other related issues. Although research has advanced our understanding of the underlying etiology of GRIN-NDD, its rarity means that significant gaps remain in knowledge about its progression over time. To address this, a hybrid decentralized study is currently enrolling individuals with GRIN-NDD to monitor their disease trajectory and inform the development of more effective, disease-specific therapeutic strategies.
GRIN-related neurodevelopmental disorder (GRIN-NDD) arises from genetic variants that alter the function of NMDA receptors. These functional changes span a spectrum – from gain-of-function (GoF) to indeterminant and loss-of-function (LoF) – as determined through a range of functional assays. While initial safety and efficacy data for radiprodil in treating GRIN-NDD GoF variants were demonstrated in the Honeycomb trial and are being further evaluated in the Phase 3 Beeline trial, emerging mechanistic evidence suggests that LoF and interminant variants may also disrupt the excitatory/inhibitory balance in neural circuits in ways that could be responsive to radiprodil modulation. GRIN Therapeutics intends to further explore ways to further investigate this possibility.
TSC / FCD type II

Tuberous Sclerosis Complex (TSC) is a rare, neurodevelopmental disorder caused by mutations in the TSC1 or TSC2 genes, leading to benign growths known as tubers throughout the body. When these tubers form in the brain, they can trigger seizures and other neurological symptoms, including behavioral dysregulation and developmental delay.
Focal cortical dysplasia type II (FCDII) is a rare, neurodevelopmental disorder caused by abnormal organization in the brain. It is frequently associated with treatment-resistant seizures, as well as other non-seizure symptoms.
Studies of affected brain tissue from individuals with TSC and FCDII have revealed alterations in NMDA receptor activity, including changes in GluN2B expression. GRIN Therapeutics is currently enrolling participants in Astroscape, a Phase 1b/2a, open-label clinical trial evaluating radiprodil in children diagnosed with TSC or FCDII.
For additional information about the Astroscape trial, visit astroscapestudy.com or clinicaltrials.gov.