Targeting NMDA Dysregulation to Treat Challenging Neurodevelopmental Disorders
Extensive research has confirmed the role of the N-methyl-D-aspartate (NMDA) receptor in the regulation of physiological functions including synaptic plasticity, long-term potentiation, learning, and memory. In the brain and nervous system, the activity of the NMDA receptor is tightly regulated, and excessive activation is associated with the clinical manifestations in several neurodevelopmental syndromes.
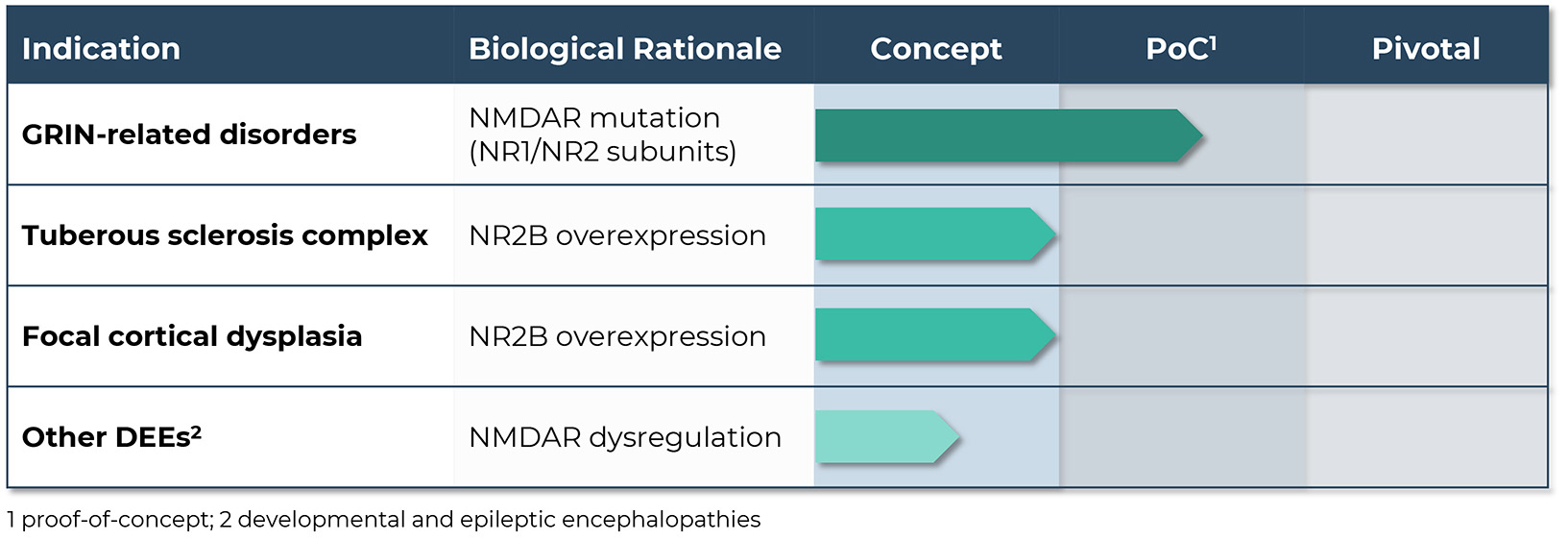
Building on our deep understanding of NMDA biology and the ability to modulate the activity of the NMDA receptor subunit NR2B, GRIN Therapeutics is advancing a targeted approach to the potential treatment of GRIN-related disorders and other pediatric neurodevelopmental disorders.
Precision modulation of the NMDA receptor presents an opportunity for development of targeted therapeutics with potential utility in the treatment of neurodevelopmental disorders including GRIN-related disorders, tuberous sclerosis complex (TSC) and focal cortical dysplasia (FCD). Radiprodil is an investigational oral, selective, negative allosteric modulator of the NR2B subunit of the NMDA receptor.
Progress in Pipeline Development
Radiprodil Phase 1B Currently Enrolling in Europe
Primary
Objectives
- Evaluate safety and tolerability of radiprodil
- Identify the optimal close range for future studies
- Determine pharmacokinetics (pk) and plasma exposure of radiprodil
Key Secondary
Objectives
- Measure the impact of radiprodil on:
- Seizure burden
- EEG
- Behavioral symptoms
- Sleep
- Motor function
- Caregiver burden
- Global impression of effect
Key Inclusion /
Exclusion Criteria
- Confirmed gain-of-function (GoF) variant
- Age between 6 months and 12 years of age
- For seizure cohort:
- At least 1 observable seizure per week
- Seizures did not improve after two mediations
- For behavioral cohort:
- Significant behavioral symptoms
Targeting Other Neurodevelopmental Disorders
Based on its observed mechanism of action, radiprodil could also have potential utility in the treatment of other neurodevelopmental disorders associated with abnormal expression of NMDA receptors, including TSC and FCD.
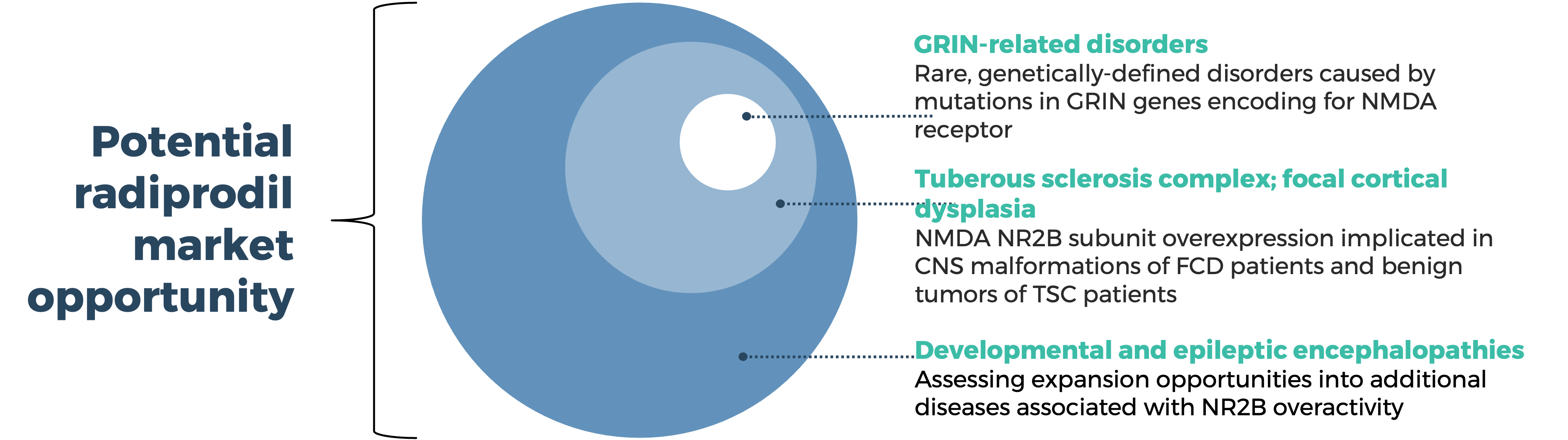
With the ability to target the NR2B-subunit of the NMDA receptor, radiprodil has the potential to target the pathogenesis of malformation of cortical development (MCD)-associated epilepsies including TSC and FCD. It has been shown to have anticonvulsant activity in a range of epilepsy and seizures models and in models characterized by enhanced NMDA signaling. Möddel et al showed increased NR2B expression in dysplastic cortex resected from drug resistant epilepsy patients correlated with the presence of prolonged ictal-like epileptiform activity in vitro (Möddel et al., 2005). The differential expression of NR2B-containing heteromeric NMDA receptors, the role of the NR2B-NMDA receptor in the generation of epileptiform activity in TSC and FCD, the positive effects of NR2B-modulation ex-vivo, and in vivo and in vitro data showing antiepileptic effects of radiprodil all indicate that it has the potential to control seizures in these patient populations.

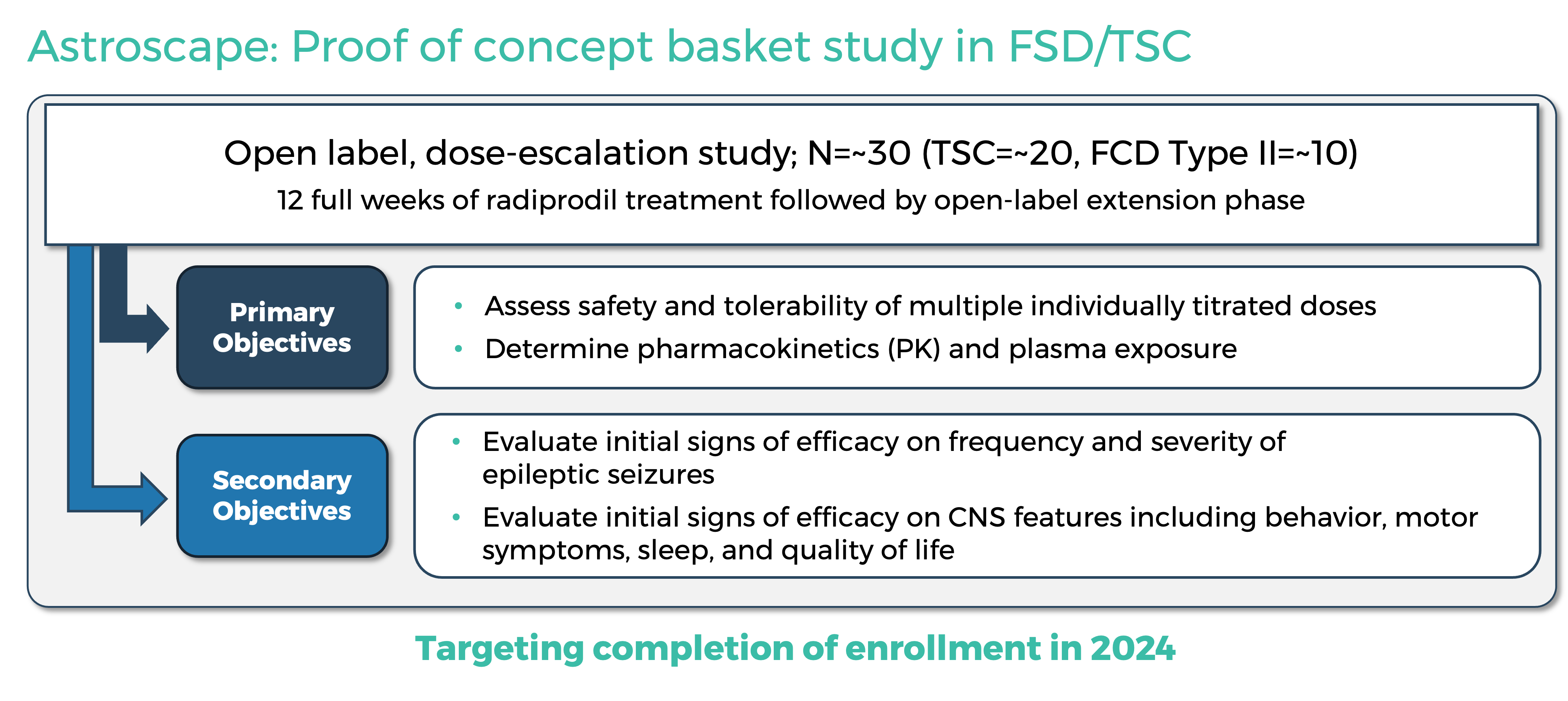
About the Astroscape Study
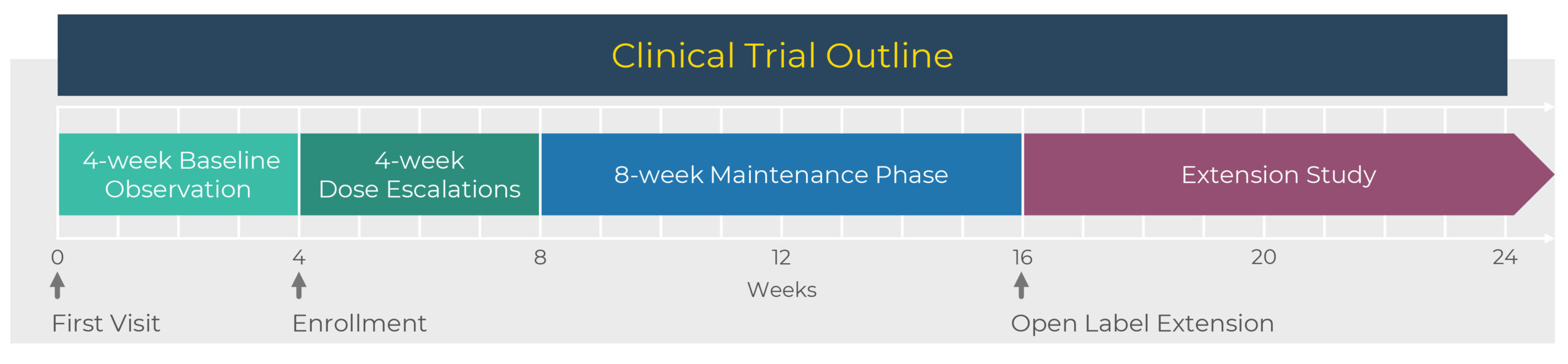
GRIN Therapeutics is advancing the Astroscape study, an open-label proof of concept basket study to assess the safety and tolerability of radiprodil at multiple dose levels in the treatment of FCD Type II and TSC.